Abstract
Introduction: Gemtuzumab ozogamicin (GO), a CD33-antibody conjugated to DNA damaging cytotoxin-calicheamcin is a re-emerging and promising drug in AML treatment. Promising results from multiple clinical trials demonstrated GO improved outcomes in both adult and pediatric patients with most pronounced effect in favorable-risk cytogenetics. In light of these results GO was reapproved for treatment of AML by FDA in Sep 2017. Given the anticipated increase in GO use post approval, it is very timely to understand mechanisms contributing to differences in patient response. Calicheamicin releases intracellularly and causes DNA strand breaks which leads to apoptosis and cell death. The anti-leukemic effects of GO are thus entirely due to calicheamicin-induced DNA damage. Given that calicheamicin is a substrate of drug transporter ABCB1 (Pgp1), genetic polymorphisms in ABCB1 can impact the intra-cellular accumulation of calicheamicin and thus clinical outcome. Our group have recently shown that ABCB1 synonymous SNP rs1045642 C>T (also well known as 3435C>T) is associated with clinical outcome in children with AML trial, now we report the results of the in vitro evaluation of impact of ABCB1 SNP on calicheamicin mediated cell cytotoxicity.
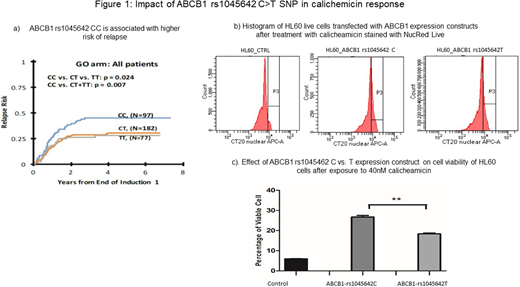
Methods: To understand differences in in vitro calicheamicin toxicity mediated by ABCB1 variation, HL-60 cells with low native ABCB1 expression were transfected with expression plasmid of ABCB1*1 (rs1045642-C and also WT for other two other SNPs in ABCB1) (WT Plasmid), and rs1045642-T. Cells were harvested 48 hours' post-transfection and treated with 40nM calicheamicin for 24 hours. Posttreatment cell were stained with permeant nuclear stain (NucRed® 647 Live Invitrogen) that emits bright far-red fluorescence upon binding to DNA in live cells. One-hour after incubation with the dye, calicheamicin toxicity was assessed by checking cell viability using flow cytometry (BDTM LSR II) and Nikon fluoresce microscope for additional visualization.
Results: As shown in Figure 1a, our previous data show rs1045642 a synonymous coding change in ABCB1, in patients treated with GO presence of variant allele (CT/TT) is associated with significantly improved outcome as compared to patients with CC genotype (risk of relapse CC=45±10%, CT=30±7% TT=28±10%, CC vs. CT/TT p=0.007). Consistent with these results we observe significant decrease in cell viability with transfection with ABCB1-rs1045642/3435T as compared to the ABCB1-rs1045642/3435-C (P < 0.05) expression constructs. These results indicate that higher levels of calicheamicin toxicity in ABCB1-3435T transfected cells as opposed to cells transfected with the WT plasmid (Figure 1b and c) probably due to impact of T allele on ABCB1 levels. rs1045642-T allele has been shown to be associated with lower ABCB1 expression in several studies and with higher expression in one study. Hofmeyer et al has previously inked TT genotype of rs1045642 with lower expression and activity of ABCB1 which is consistent with our observation with respect to calicheamicin. Although more work is required to establish the clear relationship between ABCB1 genotypes and calicheamicin efflux, our results suggest that presence of ABCB1- low expressing rs1045642 TT genotype might be resulting in lower-efflux and thus higher intracellular abundance of calicheamicin in leukemic cells which in turn makes cells more sensitive to calicheamicin.
Acknowledgments: Funding support from R21CA155524 and Children's Oncology group
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal